QCM-D analysis helps understand protein adsorption - aggregation relation
One of the primary hurdles in pharmaceutical development revolves around protein aggregation, a phenomenon with the potential to compromise both the quality and safety of drugs. A recent study sheds light on the intricate connection between protein adsorption and aggregation at the oil-water interface, offering valuable insights for crafting more robust therapeutic formulations. Among the analytical methods employed, QSense QCM-D played a pivotal role in unraveling this complex puzzle.
Monoclonal antibodies in prefilled syringes could interact with silicone-oil coated surfaces
Interactions between monoclonal antibodies and silicone-oil coated surfaces in prefilled syringes As discussed in a prior blog post, prefilled syringes are a prevalent means of storing and administering therapeutic proteins, often featuring interior surfaces coated with silicone oil to ease syringe use. The interaction of therapeutic proteins with this hydrophobic surface can lead to adsorption, a suspected contributor to aggregation.
Analyzing adsorption and aggregation of monoclonal antibodies at silicone oil–water interfaces
Investigating adsorption and aggregation of monoclonal antibodies at silicone oil–water interfaces In order to deepen comprehension of protein interaction with silicone-oil coated surfaces and its potential correlation with aggregation, a team of researchers conducted an analysis of the surface behavior of monoclonal antibodies (mAbs), a key class of protein-based therapeutics used in treating various diseases. The study also explored whether two commonly used surfactants could mitigate the adsorption and aggregation of the mAbs under scrutiny.
Using QCM-D to analyze surface mass adsorption
In order to fully grasp the dynamics of surface activity and monoclonal antibody (mAb) aggregation, a comprehensive approach incorporating multiple analysis methods was undertaken. Specifically, the surface mass adsorption of the two mAbs, the two surfactants, and combinations of mAb with surfactants was examined using QCM-D, a cutting-edge technology capable of detecting time-resolved mass alterations at the nanoscale level, thereby providing valuable insights into these complex interactions.
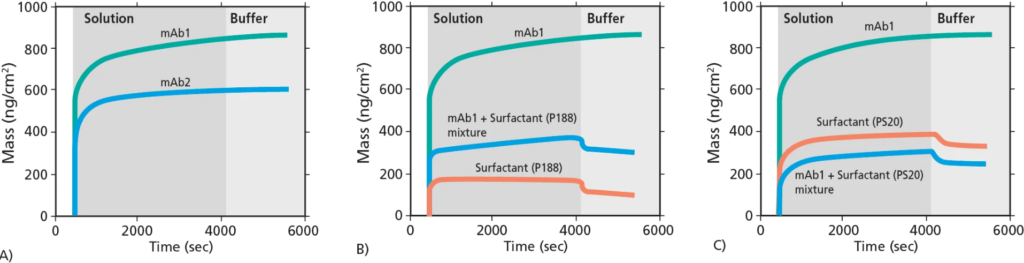
Figure 1. Mass uptake at the sensor surface measured by QCM-D. A) Comparison of mass uptake of mAb1 and mAb2. B) and C) comparison of mAb1, surfactants P188 and PS20, and mAb1 + surfactant mixtures.
Surface mass uptake results
The QCM-D analysis yielded significant insights into the interfacial adsorption and surface mass uptake of monoclonal antibodies (mAbs), surfactants, and their mixtures. For instance:
- mAb1 exhibited higher adsorption compared to mAb2.
- Initial adsorption of mAbs was rapid, followed by saturation at distinct levels for each mAb.
- Under the explored conditions, mAbs adsorption proved essentially irreversible.
- Surfactant adsorption, on the other hand, demonstrated partial reversibility.
- In certain mAb1 + surfactant mixtures, both the mAb and surfactant co-adsorbed, while in others, the interface was predominantly occupied by surfactants.
Integrating these findings with complementary characterizations, the researchers inferred a direct correlation between mAbs adsorption at the oil-water interface and aggregation. Furthermore, the study underscored the role of surfactants in mitigating mAb aggregation by competitively adsorbing to the interface.
